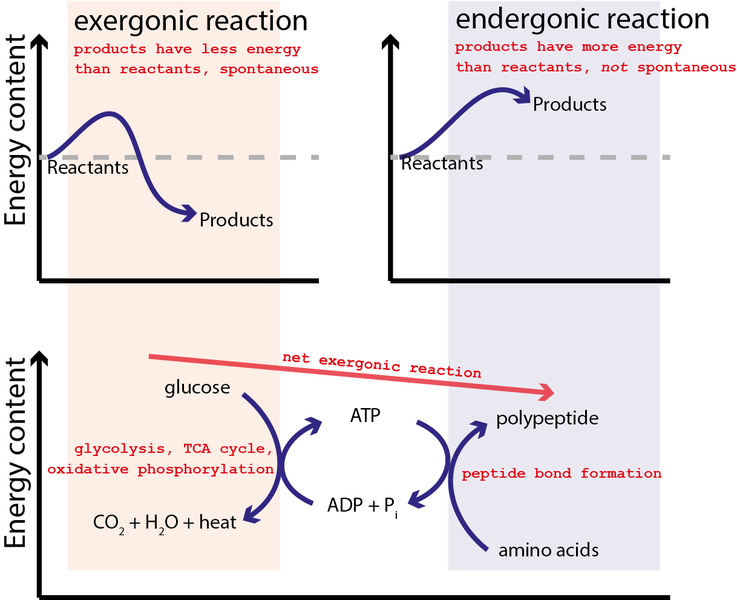
An endergonic reaction occurs by coupling with an even more exergonic. In exergonic reaction the free energy of the products is lower than that of.
Atp Cycle And Reaction Coupling Energy Article Khan Academy
Energy coupling of endergonic and exergonic reactions within cells a.

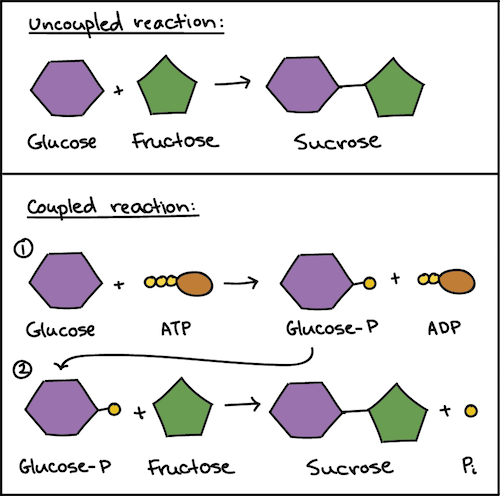
. The key to coupling exergonic and endergonic reactions is the formation of this phosphorylated intermediate which is more reactive less stable than the original unphosphorylated molecule. Enzymes can couple exergonic reactions with endergonic reactions to result in a coupled reaction that is exergonic overall. Enzymes can couple exergonic reactions with endergonic reactions to result in a coupled reaction that is exergonic overall.
Exergonic and endergonic reactions. Utilizes ATP to carry energy between the d. This means that the energy an exergonic process release is used to enable an endergonic process.
Solution for Coupling Endergonic Exergonic Reactions in Metabolism Hesss Law. Exothermic reaction When energy is released in a chemical reaction it is called an exergonic reaction. The energy for an endergonic reaction comes from a n _____ reaction.
The ATP molecule is the key to energy coupling. Used to drive another exergonic reaction. You have to pick the.
The cellular processes of energy intake and output are called endergonic or exergonic. Endergonic exergonic exothermic and endothermic reactionsWatch the next lesson. Exergonic and endergonic reactions result in changes in Gibbs free energy.
The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. Oxidation-reduction redox reactions are examples of the coupling of. The formation and hydrolysis of ATP constitute what might be called an energ y-coupling cycle in which ADP picks up energy from exergonic reactions to become ATP which then donates energy to endergonic reactions.
An endergonic reaction occurs by coupling with an even more exergonic reaction. In cells this process generally occurs in one of two ways either through the transfer of electrons or the transfer of a phosphate group. Used to drive an endergonic reaction.
Energetic coupling between exergonic and endergonic reactions allows free energy released from one reaction to drive another. Lost as nonusable heat to the environment. The linking often happens through a shared intermediate meaning that a product of one reaction is picked up and used as a reactant in.
Permits biological reactions to proceed at temperatures consistent with life. What is the fate of the phosphate group that is removed when ATP is converted to ADP. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need.
In this lab you will create an endothermic and an exothermic reaction. In most cases cells use a strategy called reaction coupling in which an energetically favorable reaction like ATP hydrolysis is directly linked with an energetically unfavorable endergonic reaction. It simply refers to the process of using an exergonic process to facilitate an endergonic process.
All of the choices are correct. An exergonic reaction is one in which the energy level of the products is lower than the energy level of the reactants a spontaneous reaction. One example of an exergonic reaction is cellular respiration in both plants and animals.
Coupling occurs when the energy released by an exergonic reaction is. In cells what is usually the immediate source of energy for an endergonic reaction. The key to coupling exergonic and endergonic reactions is the formation of this phosphorylated intermediate which is more reactive less stable.
Energy coupling is the cells ability to use the energy released from exergonic reactions is to drive endergonic reactions. An exergonic reaction is one in which the energy level of the products. Use the appropriate AG values below from the table.
ATP is the common component of these reactions and is the agent of coupling as illustrated in Figure 86. All of the choices are correct. Uses heat released by one reaction to fuel the other reaction.
When we talk of energy coupling it refers to the process of energy transfer from a catabolism reaction to an anabolism chemical reaction. Used to decrease the entropy of the universe. Enzymes can couple exergonic reactions with endergonic reactions to result in a coupled reaction that is exergonic overall.
Energy coupling of endergonic and exergonic reactions within cells permits biological reactions to proceed at temperatures consistent with life uses heat released by one reaction to fuel the other reaction and utilizes ATP to carry energy between the exergonic and endergonic reactions. An exergonic reaction is one in which the energy level of the products is lower than the energy level of the reactants a spontaneous reaction. The reaction ADP P -- ATP is a n _____ reaction.
An exergonic reaction is one in which ΔG increases and an endergonic process is one in which ΔG decreases.
9 6 Coupled Reactions Chemistry Libretexts

Endergonic And Exergonic Reactions Feedback Inhibition Youtube


0 Comments